Systemic administration of MPTP to C57bl/6 mice induces a rapid degeneration of dopaminergic neurons within the substantia nigra. This robust and well-characterised model is amenable to a variety of disease-modification study designs and allows for rapid evaluation of test compounds.
- Well characterised mouse model of Parkinson’s disease
- Robust and reproducible model
- Allows for rapid evaluation of disease-modifying potential of test compounds
Model Overview
Various iterations of the MPTP-lesioned mouse are offered by Atuka. In our most commonly used model, MPTP (25 mg/kg, i.p.) is administered to C57bl/6 mice once daily for 5 days. This leads to a loss of tyrosine hydroxylase positive neurons in the substantia nigra that is stable over a period of weeks. Test compounds can be administered before MPTP (neuroprotective) or after MPTP (neurorestorative).
MPTP induces a robust and stable dopaminergic lesion
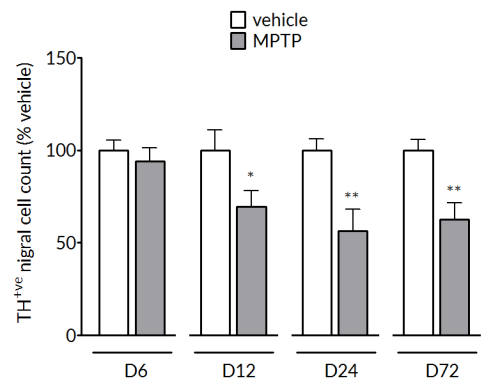
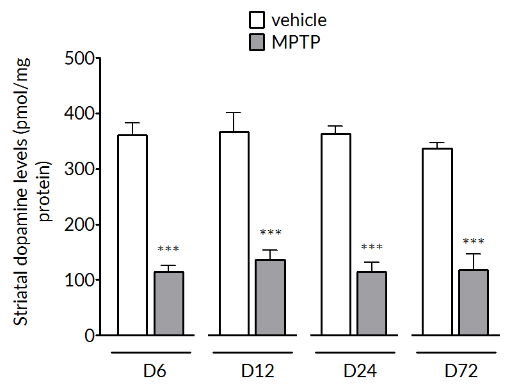
MPTP produces a significant reduction of tyrosine hydroxylase positive cells in the substantia nigra (left) and striatal dopamine levels (right). The loss in striatal dopamine is maximal by Day 6 and is stable up to Day 72. The loss in TH+vecells is slower to develop and is maximal by Day 24 and maintained out to Day 72.
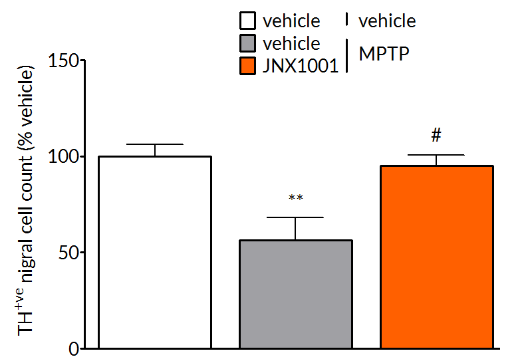
MPTP-induced deficits are reversed by pharmacological treatment.
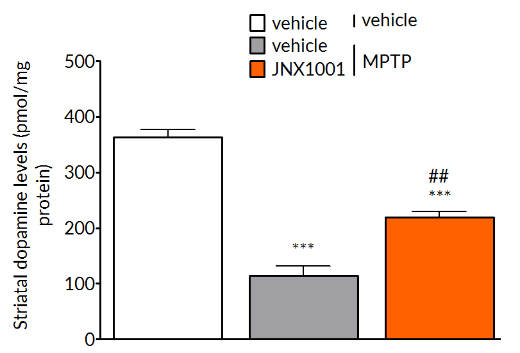
MPTP-induced reductions in tyrosine hydroxylase positive cells in the substantia nigra (left) and striatal dopamine levels (right) are prevented by treatment with JNX1001, a compound that increases GDNF and BDNF production.
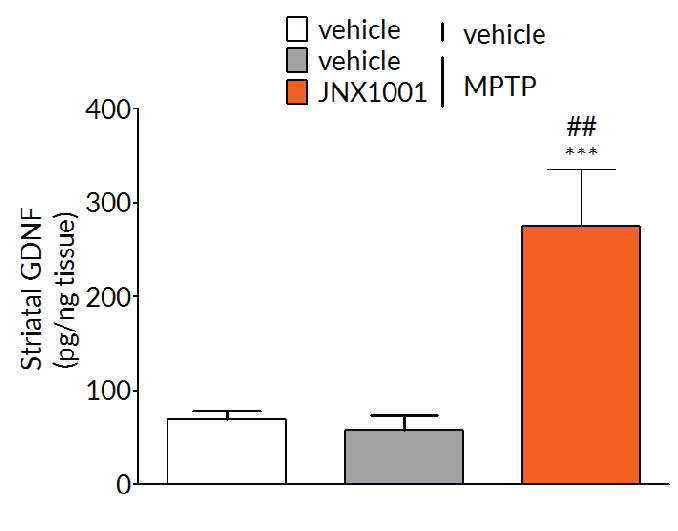
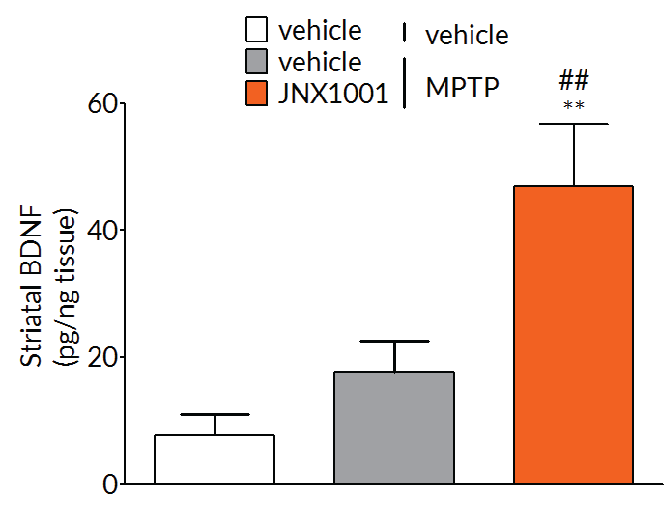
Client-specified endpoints allow demonstration of target engagement.
In MPTP-lesioned mice, JNX1001 was demonstrated to increase striatal GDNF and BDNF levels demonstrating that JNX1001 was engaging its proposed mechanism of action and providing a rationale behind its protective and restorative effects.
Experimental readouts
- Post-mortem – Routine post mortem analyses include striatal dopamine and dopamine transporter (DAT) levels, striatal α-synuclein expression and the number of number of TH+vecells in the substantia nigra. Additional post-mortem measures can be incorporated at the request of the client.
- Target engagement – Demonstration of target engagement can often be incorporated into the study design aiding translation from rodent studies to non-human primate studies and ultimately to clinical studies.
- Imaging – We offer both MRI and PET imaging that allows longitudinal measurement of markers of dopaminergic function and metabolism.
- Pharmacokinetics – Can be incorporated into all studies. Blood and CSF can be sampled throughout the study and terminal samples of brain and other tissues can be collected.