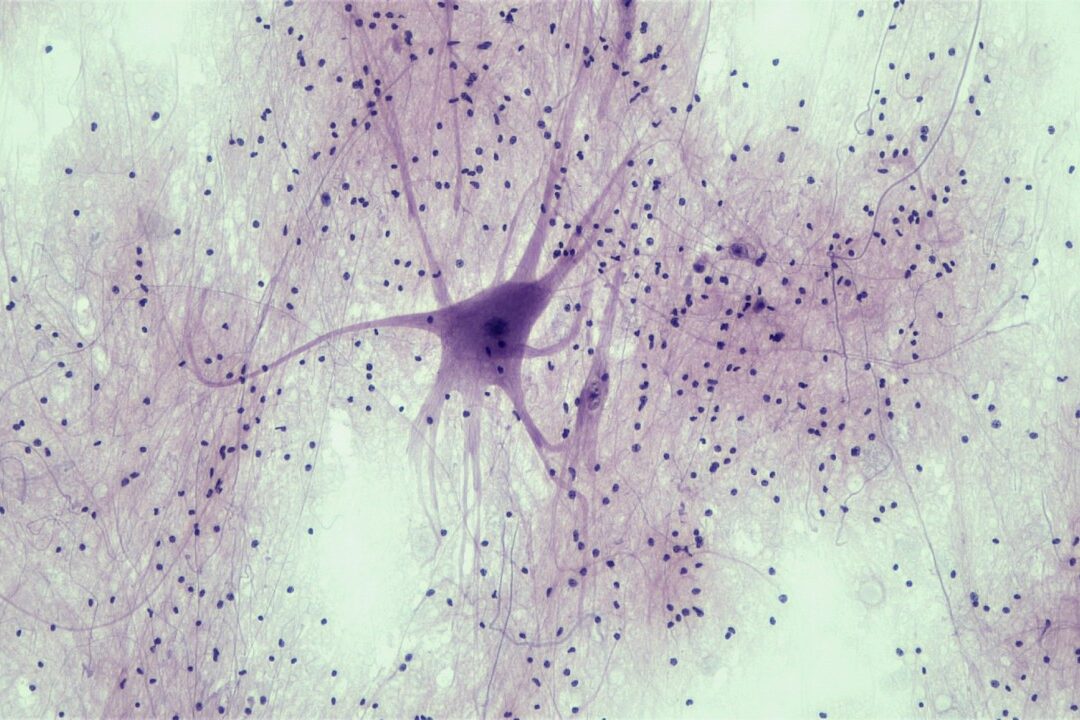
α-Synuclein is the major constituent protein of aggregates in Lewy bodies, the pathological hallmark of Parkinson’s disease. Viral delivery of α-synuclein to the substantia nigra of rats or mice produces a slowly developing degeneration of dopaminergic neurons in the substantia nigra with behavioural deficits and molecular pathology analogous to that observed in people with Parkinson’s disease.
- Disease-relevant mechanism of dopaminergic degeneration
- Can be used to assess disease modifying therapies
- Slowly developing rodent model of Parkinson’s disease
Model Overview
AAV1/2-hA53T-α-synuclein vectors are injected unilaterally into the substantia nigra of rats or mice using stereotaxic techniques. Degeneration of dopaminergic neurons occurs over several weeks allowing test compounds to be evaluated for disease modifying potential.
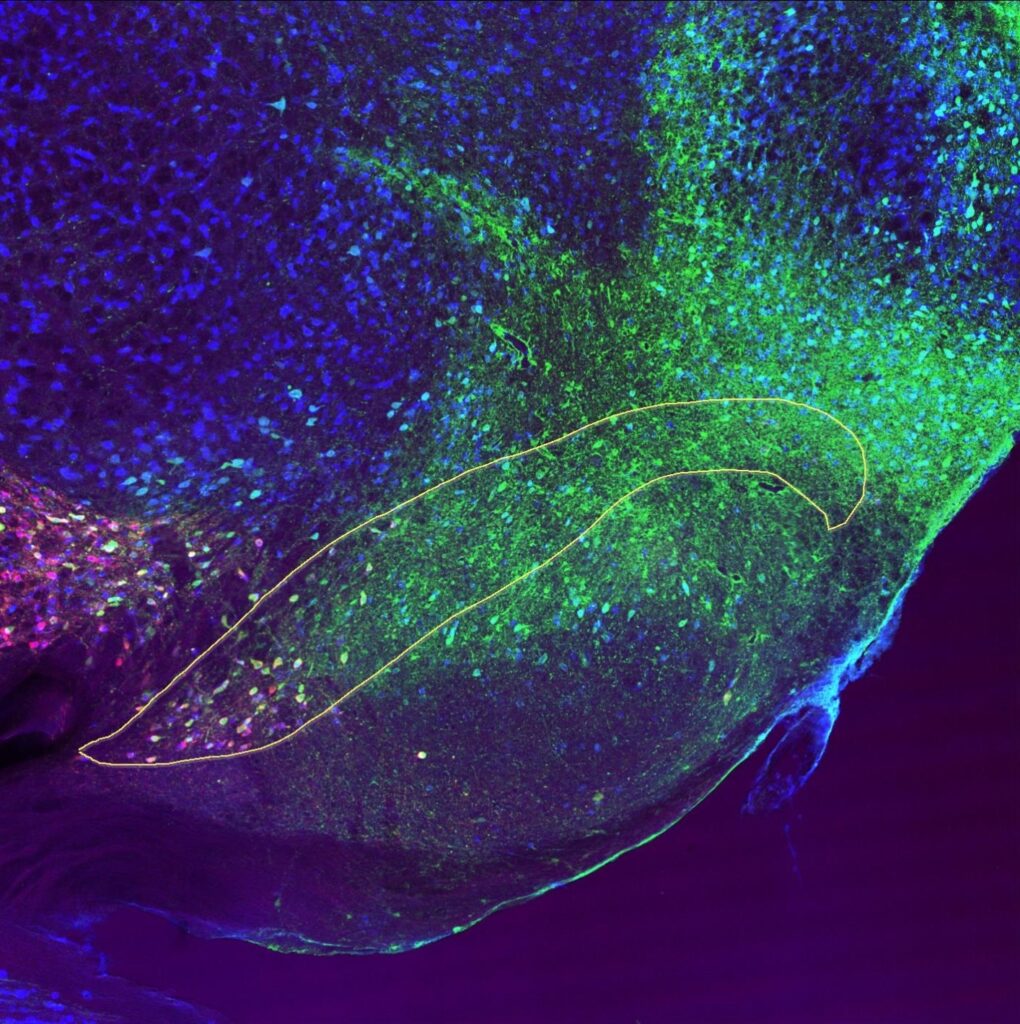
Slice through the rat brain showing the substantia nigra (marked by the yellow line) that has been stained with markers for three different proteins; green = α-synuclein, red = dopaminergic neurons, blue = all neurons.
- Cells staining cyan = co-localisation of α-synuclein stain + neuronal stain
- Cells staining magenta = co-localisation of dopaminergic neuron stain + neuronal stain
- Cells staining yellow = co-localisation of α-synuclein stain + dopaminergic stain
- Cells staining white = co-localisation of α-synuclein stain + dopaminergic neuron stain + neuronal stain
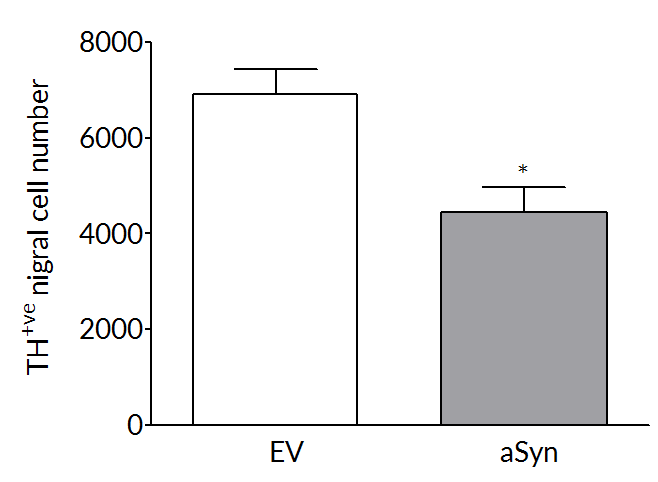
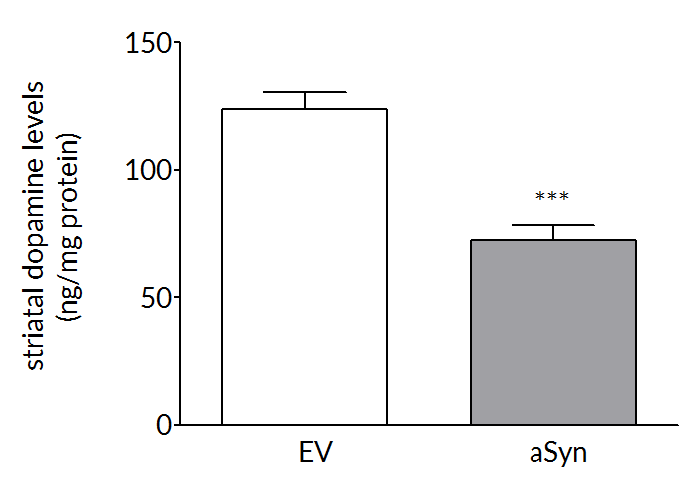
Robust loss on dopaminergic endpoints
Dopaminergic cell loss develops over time so that at 6 weeks post-surgery there is a 35-45% loss of striatal dopamine (right) and tyrosine hydroxylase positive cells (left) in the substantia nigra.
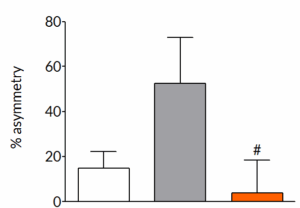
AAV-α-synuclein produces forelimb asymmetry that can be prevented by pharmacological intervention
Six weeks post-surgery, rats exhibit a pronounced forelimb asymmetry. Once daily treatment with trehalose, a known autophagy enhancer, completely prevents the development of forelimb asymmetry. This demonstrates that the model is able to identify compounds that can slow the progression of symptoms in the model.
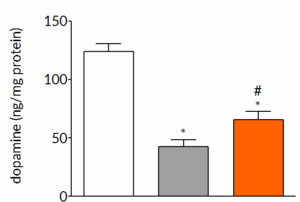
AAV-α-synuclein-induced loss of dopamine can be prevented by pharmacological intervention
Six weeks post-surgery, rats exhibit a pronounced loss of striatal dopamine. Once daily treatment with trehalose significantly reduces the striatal dopamine loss demonstrating that the underlying pathological changes that occur in the model can be modified by pharmacological intervention.
Experimental readouts
- Behavioural – Behaviour is assessed by a cylinder test that examines forelimb asymmetry. The limb asymmetry indicates an imbalance in striatal dopaminergic function between the side injected with AAV1/2-A53T-α-synuclein and the contralateral side.
- Post-mortem – Routine post-mortem analyses include striatal dopamine and dopamine transporter (DAT) levels, striatal α-synuclein expression and the number of number of tyrosine hydroxylase positive cells in the substantia nigra. Additional post-mortem measures can be incorporated at the request of the client.
- Target engagement – Demonstration of target engagement can often be incorporated into the study design aiding translation from rodent studies to non-human primate studies and ultimately to clinical studies.
- Imaging – We offer both MRI and PET imaging that allows longitudinal measurement of markers of dopaminergic function and metabolism. Imaging can also be used to demonstrate target engagement.
- Pharmacokinetics – Can be incorporated into all studies. Blood can be sampled throughout the study and terminal samples of CSF and brain and other tissues can be collected.