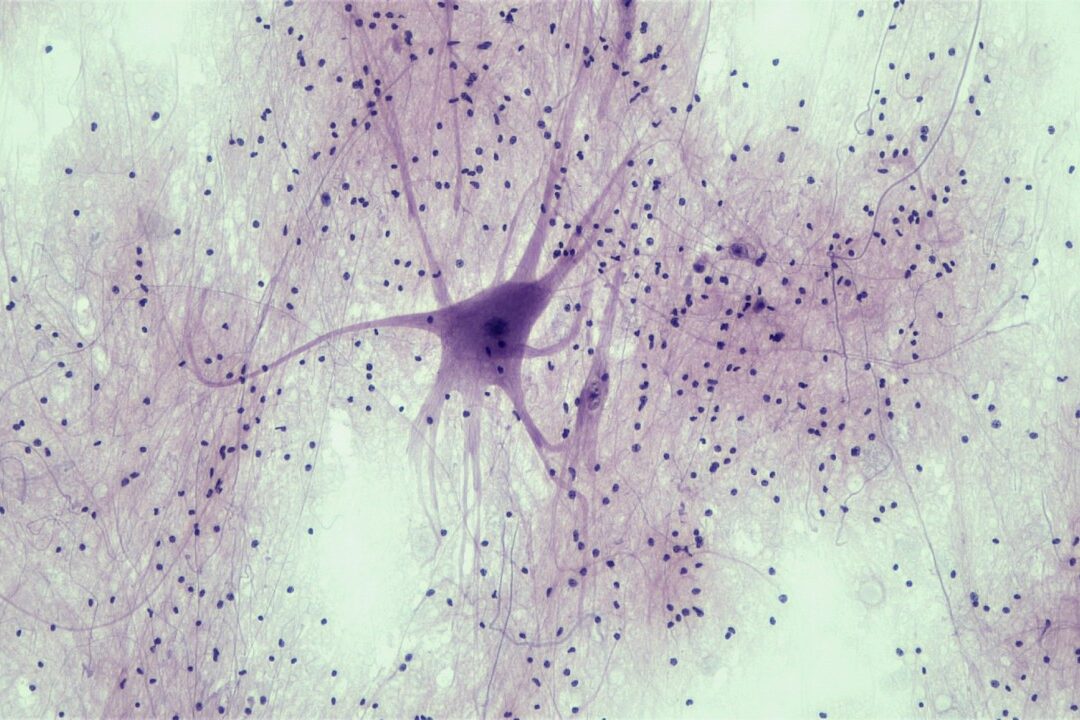
α-Synuclein is the major constituent protein of aggregates in Lewy bodies, the pathological hallmark of Parkinson’s disease. Viral delivery of α-synuclein to the substantia nigra of rats or mice produces a slowly developing degeneration of dopaminergic neurons in the substantia nigra with behavioural deficits and molecular pathology analogous to that observed in people with Parkinson’s disease.
- Disease-relevant mechanism of dopaminergic degeneration
- Can be used to assess disease modifying therapies
- Slowly developing rodent model of Parkinson’s disease
- Allows generation of fixed and fresh frozen midbrain samples
Model Overview
AAV1/2-hA53T-α-synuclein vectors are injected bilaterally into the substantia nigra of rats or mice using stereotaxic techniques. Degeneration of dopaminergic neurons occurs over several weeks allowing test compounds to be evaluated for disease modifying potential. Tissue from one hemisphere can be fixed to allow counting of TH+ve cells in the substantia nigra and pSer129 aSyn pathology assessment in the striatum whilst the second hemisphere can be used to analyse striatal dopamine and DAT, midbrain α-synuclein levels, or other endpoints requiring fresh-frozen tissue, e.g., proteomics. Behavioural changes can be detected in an Open Field Arena
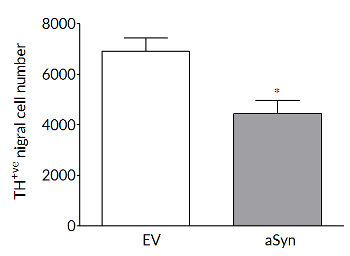
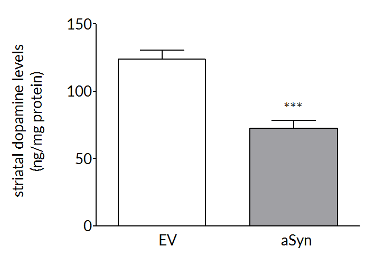
Robust loss on dopaminergic endpoints
Dopaminergic cell loss develops over time so that, in rat, at 6 weeks there are significant losses in TH+ve cells in the substantia nigra (left) and striatal dopamine levels (right).
Bilateral AAV-α-synuclein produces a decrease in distance travelled in the Open Field Arena (OFA)
Rats exhibit a progressive reduction in distance travelled in an OFA test. Further analysis demonstrates that this reduction is due to the rats being less mobile, with the average speed when they are moving being unchanged.
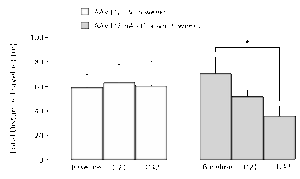
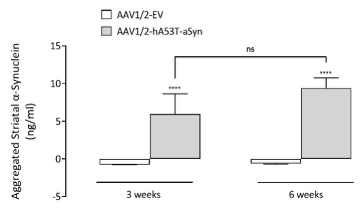
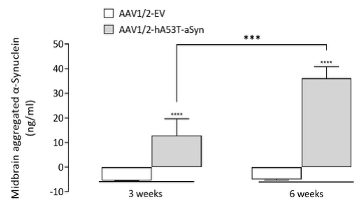
Bilateral AAV-α-synuclein induces an increase in aggregated α-synuclein in both the midbrain and striatum
At both 3- and 6-weeks post-surgery, aggregated α-synuclein can be detected in both the striatum and midbrain. Interestingly, whilst striatal levels of aggregated α-synuclein levels are similar at 3- and 6-weeks, levels of aggregated α-synuclein in the midbrain continue to rise. This demonstrates the advantage of having fresh-frozen midbrain tissue for post-mortem analyses.
Experimental readouts
- Behavioural – Behaviour is assessed by OFA. Distance travelled is reduced by ~50% in the AAV-α-synuclein group compared to the AAV-Empty Vector group. Detailed analyses, including number of freezing episodes, time mobile, and average speed can be assessed.
- Post-mortem – Routine post-mortem analyses include striatal dopamine and dopamine transporter (DAT) levels, striatal and midbrain α-synuclein expression (total and aggregated) and the number of number of tyrosine hydroxylase positive cells in the substantia nigra. Additional endpoints can be incorporated at the request of the client. The model generates both fixed and fresh-frozen tissue, thus providing flexibility on what endpoints can be assessed.
- Target engagement – Demonstration of target engagement can often be incorporated into the study design aiding translation from rodent studies to non-human primate studies and ultimately to clinical studies.
- Imaging – We offer both MRI and PET imaging that allows longitudinal measurement of markers of dopaminergic function and metabolism. Imaging can also be used to demonstrate target engagement.
- Pharmacokinetics – Can be incorporated into all studies. Blood can be sampled throughout the study and terminal samples of CSF and brain and other tissues can be collected.