Available for use in both new (marmoset) and old-world (macaque) primates, MPTP induces degeneration of dopaminergic neurons in the substantia nigra which translates to a behavioural impairment similar to that observed in people with Parkinson’s disease. Repeated administration of L-DOPA induces dyskinesia and other motor complications, as is seen in the clinic.
- Well characterised primate model of L-DOPA-induced dyskinesia
- Excellent face validity allows application of modified clinical rating scales
- Highly predictive of Phase II clinical efficacy
- Rapid evaluation of test compounds
Model Overview
Marmosets or macaques, previously rendered parkinsonian by MPTP administration, are administered twice-daily L-DOPA (25 mg/kg/day p.o.) over a 12-week period until the development of dyskinesia. Stable and robust dyskinesia can be maintained over several years by regular administration of L-DOPA. The model can be applied to examine effects of treatment on the development of dyskinesia or dyskinesia once established.
Model highly predictive of Phase II clinical efficacy
Several compound classes evaluated in the MPTP primate model of dyskinesia have also been tested in Phase II clinical studies. The dyskinetic MPTP primate has excellent face and predictive validity for treatments of dyskinesia and has been instrumental in the development of several approaches that are currently used to treat motor complications in PD.
| Compound class | NHP | Phase II |
|---|---|---|
| NMDA receptor antagonists | Yes ✅ | Yes ✅ |
| mGlu5 NAMs | Yes ✅ | Yes ✅ |
| AMPA antagonists | Yes ✅ | Yes ✅ |
| alpha adrenergic antagonists | Yes ✅ | Yes ✅ |
| 5-HT1A agonists | Yes ✅ | Yes ✅ |
| 5-HT1B/D antagonists | Yes ✅ | Yes ✅ |
| 5-HT2A antagonists/inverse agonists | Yes ✅ | Yes ✅ |
| mu opioid antagonists | Yes ✅ | N/A |
| alpha 7 nicotinic agonists | Yes ✅ | N/A |
| beta 2 nicotinic agonists | Yes ✅ | N/A |
| SV2A | Yes ✅ | No ❌ |
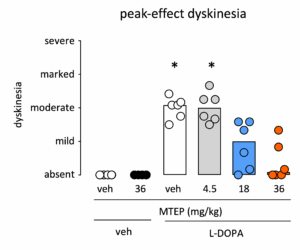
L-DOPA-induced dyskinesia can be reversed by pharmacological intervention.
L-DOPA-induced dyskinesia can be reversed by a wide range of pharmacological interventions. In this example MTEP, an negative allosteric modulator at the mGluR5 receptor, dose-dependently reduces L-DOPA induced dyskinesia.
Effect of test compounds on dyskinesia and parkinsonian symptoms can be assessed simultaneously
It is critical that compounds that reduce L-DOPA-induced dyskinesia do so without reducing the anti-parkinsonian benefits of L-DOPA. The primate model of dyskinesia allows the effect of test compounds on both dyskinesia and parkinsonian symptoms to be assessed in the same animal, thus providing a strong go/ no-go decision for continued development.
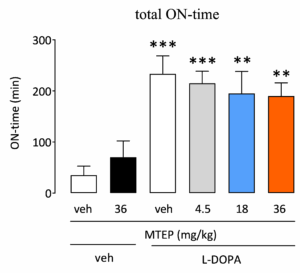
Experimental readouts
- Behavioural – Behavioural assessments include the monkey parkinsonian disability rating scale and dyskinesia rating scale, the primate equivalents of the clinical rating scales used to assess disability and dyskinesia in PD patients. Additional behavioural endpoints include home-cage activity, observation-cage activity and fine motor control.
- Post-mortem – If required, post-mortem analyses can include striatal dopamine and dopamine transporter and the number of number of tyrosine-hydroxylase positive cells in the substantia nigra. Additional post-mortem measures can be incorporated at the request of the client.
- Pharmacokinetics, safety and blood chemistry – Can be incorporated into all studies. Blood and CSF can be sampled throughout the study and functional observational battery and blood chemistry can be used to assess off-target and adverse effects.
- Imaging – We offer both MRI and PET imaging that allows longitudinal measurement of markers of dopaminergic function and metabolism.